A few more items of chem to make sure they are in your head.
First of all, you KNOW that all atoms fill up their orbitals from first to seventh orbital in order. Lowest energy to highest.
The first orbital can only hold 2 electrons, while the second can only hold eight electrons.
In the atom called Hafnium, number 72, a small quirk occurs, but the State Education Department does include an little star, an asterisk, for you to remember and use. See it in there? Right under the 72?
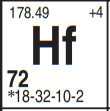
That makes you look to the bottom left of the Periodic Table, where it says:
*denotes the presence of (2-8-) for elements 72 and above
That is a nasty trick to play on new chemistry students. They’re saying they can’t “fit” that 2-8 in front of all those 18’s, and some of you got tricked wihen asked for the electron configurations on the 39 Atoms handout.
Next, counting protons, neutrons and electrons in all atoms.
The Atomic mass rounds to the nearest whole number. Take care with that. Then, subtract the atomic number, the number of protons from the mass, and you end up with neutrons. The positive protons always equals the negative electrons.
Ex: mercury is atom #80. the mass rounds to 201 amu. that means 201 protons plus neutrons. Since it’s #80 that means there are 80 protons in total. Mass 201 minus 80 protons leaves 121 left over, or 121 neutrons.
80 protons means 80 electrons too.
The decimals of the mass come in from the PROPORTIONS of the isotopes. Isotopes are chemically identical atoms, but they have different numbers of neutrons (and therefore different masses). It’s the proportions of each isotope that provide the decimals of the math.
When you round the atomic mass from the periodic table, the nearest whole number is the most common isotope.
The weighted average atomic masses are calculated from all of the NATURALLY OCCURRING isotopes, not including any man made ones (or woman made either).