
Redox stands for Reduction and Oxidation, a pair of reactions that take place at the same time, in electrochem. As the name implies, it’s about moving electrons. Electrons are “lost” or transferred in this process. They are “gained” on the other side.
The LOSS OF ELECTRONS is OXIDATION. (LEO)
The GAIN OF ELECTRONS is REDUCTION (GER)
Leo the Lion goes Ger!
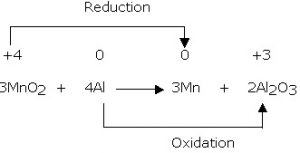
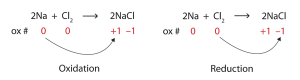
Redox is also the plating of pretty, shiny metals like gold or silver or platinum, onto strong but less beautiful metals like titanium and iron.

Finally, redox is the hydrolysis of water by electricity. Water decomposes into hydrogen gas and oxygen gas.
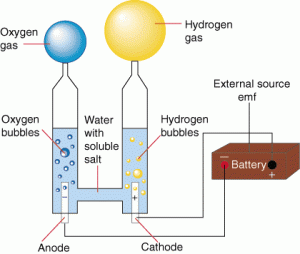
It’s all about the electrons.
Read the BASICS.