Welcome to real chemistry, finally.
This is where we will learn how to put atoms together to make new pure substances, with new properties.
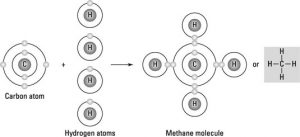
A pure substance has a formula, like…
Fe (an atom of the element iron), or
HCl (a molecule of hydrogen monochloride) or even
NaCl (a formula unit of the ionic compound table salt).
When pure substances combine, or decompose, they form new substances with new properties. Most chemical reactions will result in bonding (although some are the result of “un-bonding”).
We will learn the details of:
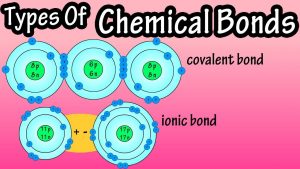
ionic bonding (cations + anions make neutral ionic compounds by transferring electrons)
Covalent bonding (two or more nonmetals share electrons when they bond)
Metallic bonding (explains how a hunk of metal stays stuck together, and most of metallic properties)
and, Inter-molecular Attractions (not really bonds, but they’re here too).
Lewis Dot diagrams, coordinate covalent bonds, resonating bonds, single, double and triple bonds, polar bonds and nonpolar bonds too.
This is going to be great.