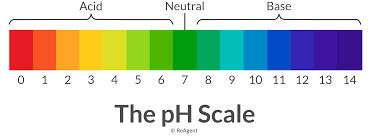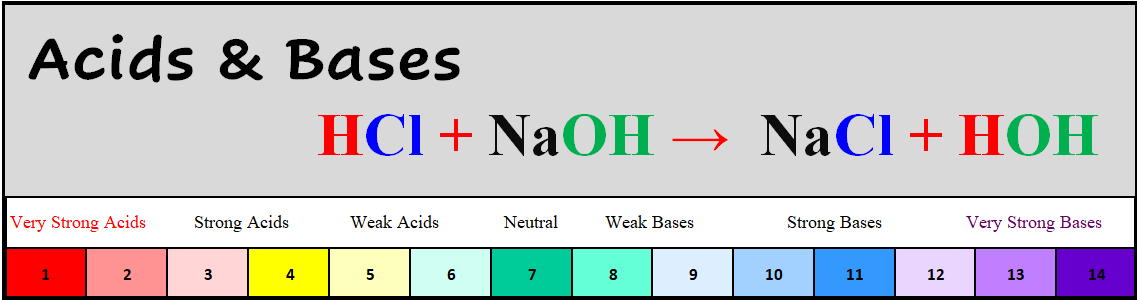
End of the 3rd Quarter is Friday April 12
Acid Base HW #1 due Wednesday 4-10 late HW NOT accepted
Acid Base HW #2 due Monday 4- 15
Acid Base HW #3 due Tues 4-16
Acid Base HW #4 due Wed 4-17
Acid Base Color Chase Lab due Fri Apr 19
Titration Classwork Due Wed April 17 NOT ACCEPTED LATE
Acid Base Titration Lab due Fri Apr 19
Eight Flash Cards to help focus your acid/base mind
Titration Practice Classwork Handout
Acid Base Indicators Handout (print white paper – before color chase)