A long, long time ago there were a bunch of elements that no one could figure out how to put into a table that made any sense.
One thought was to put them into triangular groups, or triads. It worked for some small groups, but lots of elements were sort of left out. That three was partly due to the trinity, popular in many religions.
Then, many thought eight was the right number, as in there are 8 notes in a scale, and music is wonderful. Another good idea but one that did not find overall success.
There were tables printed onto the inside of tubes, that one could look into and then turn, to see “patterns” between elements. Not very easy to use and again, not quite right.
Finally, a crazy looking (by modern standards) Russian chemist, Demteri Mendeleev went to bed one night (as the story goes) and dreamed up the basis of the modern table. He’d been playing with a set of cards he made up listing each known element and all the properties he had for each, and tried to make sense of these properties. in table form.
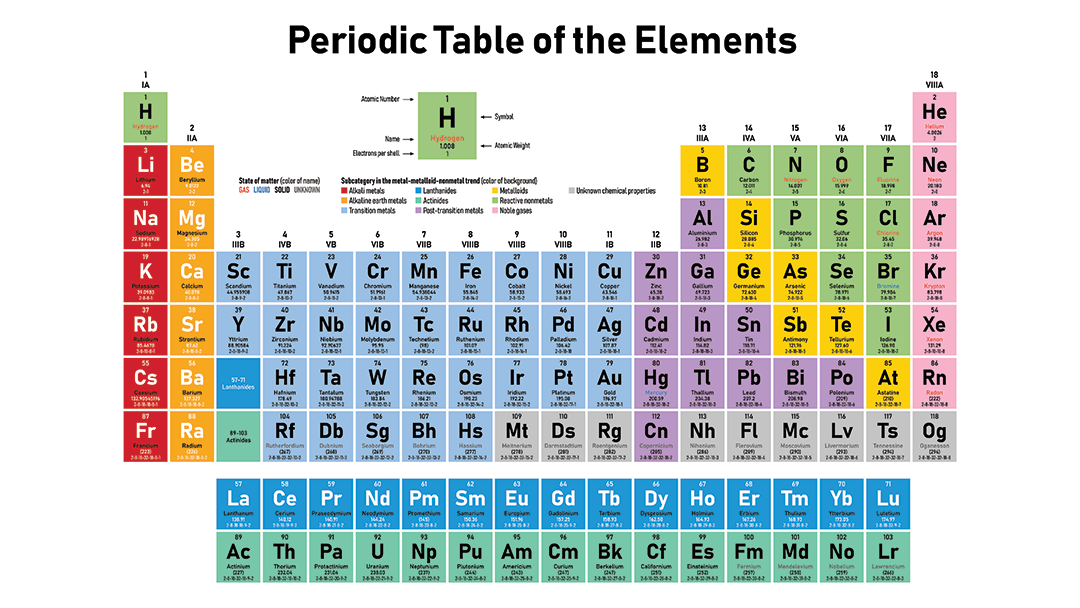
What we have now is a cool-shaped table with columns called the GROUPS, and long left to right rows, called PERIODS. Rows and columns are “normal” but the shape of this table is a big part of its mastery of organizing matter.
The Periodic LAW states that when the atoms are in the order of increasing atomic number, there will be a periodic repetition of their properties (in the groups). The Periodic part of the name means “repitition” of properties.
Atoms in group one are rather similar when compared to all other elements. They all have 1 valence electron. They all bond similarly too, because they all make +1 cations. All the group one metals bond in a 1:1 ratio with chlorine (or F, Br, or iodine. They all make a 2:1 ratio when bonding with oxygen (or S, Se, or Te).
There are many patterns to discern, data hiding out in plain site. This is going to be fun, but there are, as always, some details. Read the BASICS asap.