Matter Blog
Friday Oct 7
Today we had the gift of our graduate student teach you all about ISOPTOPE math. Isotopes are important, and here goes…
All atoms are isotopes. Each kind of atom has isotopes of itself. There are 3 isotopes of hydrogen. Copper has 29 known isotopes. Argon has less, just 26! Of the 118 known elements there are more than 1500 known isotopes. Some isotopes are naturally occurring and some scientists have created.
An isotope is a chemically identical atom that has a different number of neutral neutrons. This makes the isotopes all chemically identical, they react and bond the same way, but they have a slightly different mass.
Most of the time this makes no real difference, but we will learn more in May about radioactive isotopes. Sometimes the number of neutrons is so weird (actually the neutron to proton ratio is so out of whack from normal) that the atom is unstable. In an attempt to get more stable, it emits parts from its nucleus to change this ratio. Radioactivity comes in a variety of “flavors” and we’ll learn more about this later in the year.
Since the isotopes don’t show up in nature, say half one mass, and half another, we can’t take just a simple average. Instead we learned to do the math called WEIGHTED AVERAGE. This takes into consideration the atomic mass (in AMU) of each isotope, as well as its relative abundance (how much of all of an element is this mass).
The math is not hard, but it’s boring. So what? You don’t have to like it, you have to eat it (like meatloaf!).
Isotopes have simple symbols like Fe-56 for the iron isotope that has mass of about 56 AMU. In a calculation we’d use the ACTUAL ATOMIC MASS instead of this “about 56 AMU” number. The actual isotopic mass for Fe-56 is 55.9349 AMU. Each isotope mass is multiplied by it’s relative abundance as a decimal (38.57% becomes 0.3857 or 4.833% becomes 0.04833)
Look at these three isotopes of hydrogen…
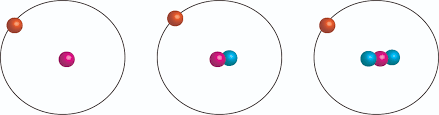
They are…
H-1, actual isotope mass of 1.007825 AMU, making up ~ 99.97% of all hydrogen.
H-2, actual isotope mass of 2.014102 AMU, making up 0.02% (approx)
H-3, actual isotope mass of 3.016049 AMU, making up 0.01% (approx)
To “do the math”, multiply the mass X the decimal proportion = partial mass, and sum the three lines.
(1.007825 AMU)(0.9997) = 1.00752… AMU
(2.104102 AMU)(0.0002) = 0.0004028… AMU
(3.016049 AMU)(0.0001) = 0.00030160… AMU
This sums up to – 1.008 AMU which is near spot on according to the periodic table value of 1.00979 AMU.
There is always some rounding on “our” end as compared to using all of the exact values with ALL of the SF that scientists have now measured to.
Adding up the decimals must sum to 1.0000 or the WHOLE amount. 0.9997 + 0.0002 + 0.0001 = 1.00000 !
Thursday Oct 6
We finished up the models of the atom today, The Rutherford (my hero) model that put a nucleus in the center, with flying electrons, further improved by Niels Bohr by putting the electrons into “orbits” (we say orbitals now, and they are not orbits), to the latest model called Wave-Mechanical model.
We also worked on the 28 Atoms Handout, where you were supposed to draw 28 Bohr models (planetary) of 28 atoms. We highlighted that isotopes exist, and that an isotope is defined as this: All atoms are isotopes, and the isotopes for one atom all have the same protons and electrons count (making them chemically identical) but different numbers of neutrons (which does give a slightly different mass but most of the time makes no other difference).
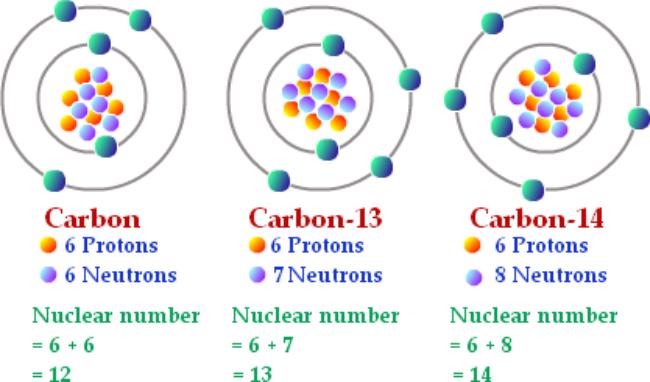
This is a picture I copied from in internet. This is not plagiarism as I am using it for educational purposes, and I am not claiming it’s mine, nor am I making money from other people’s work. Also, “nuclear number” should be “atomic mass”. I don’t know who made this nice drawing, but they clearly were NOT in my class!
The atoms of the periodic table all have a mass, and the “rounded to the nearest whole number” is the mass of the MOST COMMON ISOTOPE, but there are others. Every atom has multiple isotopes, and no one really knows why. It just is, like why do have to have mosquitos on Earth, no one knows, but we deal with this. Isotopes is a cool word too. Way better than “mitochondria” or “gravity” or even “volcano”!!!
Wednesday Oct 5
We had a great class today, discussing protons, neutrons, and electrons, and starting the history of the models of the atom. That sounds dull, but it is the history of our class. Democritus, over 2400 years ago gave us the idea of the “ultimate particle” or ATOMOS (Greek for atom). We still use that today.
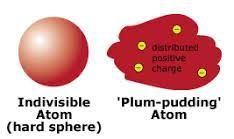
Then John Dalton came up with modern chemistry in his barn in England in the early 1800’s. JJ Thomson discovers the electron and puts that new particle into his model of the atom that looks (in his mind) like the dessert his wife bakes for him called “plum pudding” (that’s bread pudding with bits of chopped up plum) Too bad she didn’t make him chocolate chip cookies, the idea of that is easier for us non-plum-pudding-eaters!
I reminded everyone to do Matter HW #2 and Atomic HW #1. And to read the Atomic BASICS.
Tuesday Oct 4
In class today we practiced the pne counting, and then did the Review Lab #1. We examined Sulfur, Titanium, Silicon and Silver. Metals and nonmetals are the only 2 kinds of atoms really, but there are seven atoms that are also called metalloids. Metalloids are really metals or nonmetals and they are also metalloids.
I am an American but I also am Italian American. Really you can only be one thing, American or Italian. Normal people say things that are sorta true, like “Italian-American”, but that’s just friendly talk, not geopolitical proper speaking.
Nine atoms touch the staircase on the periodic table. The staircase separates the bulk of the metals from the non metals, (hydrogen is a pesky exception). If an atom touches the line it is a metal and a metalloid, OR, it’s a nonmetal and a metalloid too. Metals that touch the line have some nonmetallic properties too. Nonmetals that touch the line have some metallic properties.
There are 9 atoms that touch the line, but just 7 metalloids. The two “exceptions” are Al and Po, both metals. Aluminum and Polonium are not metalloids, they break this “rule”. How will you remember that? Well, Al and Po are not metalloids, they are the “dog food” exception.
Read the BASICS and do Atomic HW #1.
Monday Oct 3
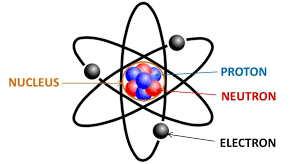
We being Atomic Theory. Atoms are made up of protons and neutrons in the nucleus, while electrons fly around the outside of this nucleus. Protons have a mass of 1 Atomic Mass Unit, and neutrons have the same mass, 1 AMU. Electrons have no mass in high school, but a real and important mass in “real life”. We are in intro chem, we “round” sometimes.
We see that the atomic mass of every atom on the periodic table is rounded to the nearest whole number in high school. The mass = the number of protons PLUS the number of neutrons. Using this fact, MASS minus ATOMIC NUMBER = the number of neutrons in an atom.
Friday Sept 30
 The end of September has arrived, we are practically at Happy Halloween!
The end of September has arrived, we are practically at Happy Halloween!
Today was the great Chemical and Physical Changes Lab, 7 experiments of fun and daring – but done safely so they only added to your knowledge base and story telling folder. I bet you thought the most exciting experiment was when the world used up some magnesium metal and some oxygen to make more magnesium oxide. It was white hot and scary, but safe too. We had a few phase changes, Solid → Liquid → Gas for dihydrogen monoxide, and one tricky one, when a Solid → Aqueous which even had a temperature change. The acronym TOPIC-B is helpful, but not all temperature changes are reactions, sometimes they are tricky phase changes.
Everyone held up well to the stress of all that work and all that yelling and the loud music, I am proud of you. Have a great weekend!
Thursday Sept 29
It was particle diagram day in chemistry today, where we learned to draw solids, liquids, and gases like cartoons, but were able to make sense of definite or indefinite volumes and shapes.
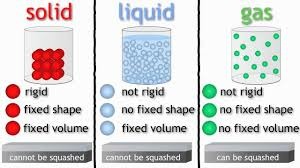
We also practiced our vocabulary: atom, element, molecule, compound, diatomic elements, etc.
A few items on the homework need clarification: Liquids are not the same as Aqueous in chemistry . Both are “wet”, but liquids are PURE SUBSTANCES in the liquid phase. They are above their own melting points, and below their own boiling points, with the particles sticky to each other (but not stuck). Aqueous means that something is dissolved into water. Not the same thing, in fact aqueous can be homogeneous but NOT PURE. Salty water is the same saltiness throughout, but it can’t be a pure substance, it is not one substance, it’s a mixture of two compounds (salt and water).
Also, M&T is a BANK, (true but this is a joke). Mercury is Hg and Tungsten is W (not M and T). The reason for this is that many elements were named in other languages. Mercury is really hydrargyrum from the Greek word for water and silver like it’s color), and Tungsten is really Wolfram, from the German (it eats up tin, like a wolf eats up sheep!).
Wednesday Sept 28
Today we reviewed how to separate mixtures from each other. Since they are NOT chemically bonded together, we use non-chemical means. We need to take advantage of a difference in their physical properties. For instance, sand and water can be separated apart using a filter. Sand particles are too big to fit through the filter paper, while the water molecules are incredibly tiny, they slide right through. If we had salty water, or sugar water, we could not use a filter, the salt and sugar particles are also too small to be caught by the filter paper.
To separate mixtures we need to take advantage of a difference in the physical properties. If there is no difference, say for size, then we need a different physical method. Say, difference in boiling points, or freezing points, or density, or if one is attracted to a magnet, or solubility in water (or not).
TOPIC-B stands for the six indicators that a chemical reaction has PROBABLY occurred. If you get a Temperature change, Odor change, Precipitate forms, chemical reactions are (spontaneously) Irreversible, Color change, or Bubbles of a New Gas, that means a chemical reaction may have happened.
Changes have to come from the chemistry, not heating with a Bunsen burner, or cooling with air conditioning. If you mix substances and they get hotter or colder, they likely reacted. If the smell changes, for the better, worse, or if it goes from no smell to smell (or opposite), it’s likely that a new substances is now present, formed during a reaction.
When mixing 2 solutions together that are homogeneous (same throughout) and all of a sudden a solid seems to “fall out” of the solution, a solid that was clearly NOT PRESENT before, that means a new substance that is not soluble in water just formed. Chemical reactions are reversible, all of them. This “irreversible” means that once the reaction occurs, it will not reverse itself by itself. It will require energy and some chem smarts to get your original reactants back. Spontaneously Irreversible didn’t fit into the acronym “TOPIC-B” as well as just “I” alone.
Color is a physical property that is a constant. It you put substances together and they change color, that means a new substance probably just formed. This is unlike painting, which is not a chemical reaction, it’s painting.
B stands for Bubbles of A New Gas. When substances combine, and react, sometimes a new product is a gas. They fizz or bubble out. If you blow bubbles of carbon dioxide into your chocolate milk at Friendly’s, that’s not the same, you are blowing bubbles, they aren’t “forming” spontaneously.
Finally, we made CHROMATAGRAPHY art. We separated the colors that are mixed in magic markers, using water. The color you see is usually made up of several “hidden” colors that your eyes register as ONE color that is a mixture of other colors. The water can pick up the soluble color particles and pull them across the paper, depending upon their size and density a short bit, or longer. The process spreads the colors out into a sort of rainbow. The water cannot pick up the sharpie (non water soluble) markers . Those colors can’t be separated with water as they are different than Crayola markers.

Tuesday Sept 27
Today I collected Matter HW#1, the Penny Density Lab is due Wednesday.
In class we covered Matter and it’s two forms: PURE and MIXED. Pure matter includes the 118 elements and ALL the millions of compounds. Compounds are substances that are made of 2 or more different kinds of elements bonded together, and which form a NEW pure substance with it’s own specific formula, and new properties that do not match or blend the properties of the reactants. Mixed matter is literally different stuff put together, but that does not chemically bond into new stuff.
We also reviewed the Law of Conservation of Matter (also known as mass). We did simple math because it is simple. The mass of the reactants always sums to the mass of the products in every single chemical reaction. You can’t lose or gain mass in a phase change either.
We went through the six phase changes too, and named them so you could memorize them. Melting and Freezing, Vaporizing and Condensing, and lastly, Subliming and Depositing.

Monday Sept 26
Today we managed to begin “Matter”, which is mostly vocabulary and the Law of Conservation of Matter, and simple math. We saw the sublimation of iodine, and learned all 6 phase changes by name. We saw that the mass of the reactants must equal the mass of the products.
I told you about my crazy fancy wedding I attended, and about my sister seeing an ET Spacecraft in the sky in Long Island (while I was at the wedding). She sent me the photo to see if she should be scared or not! (she’s fine)


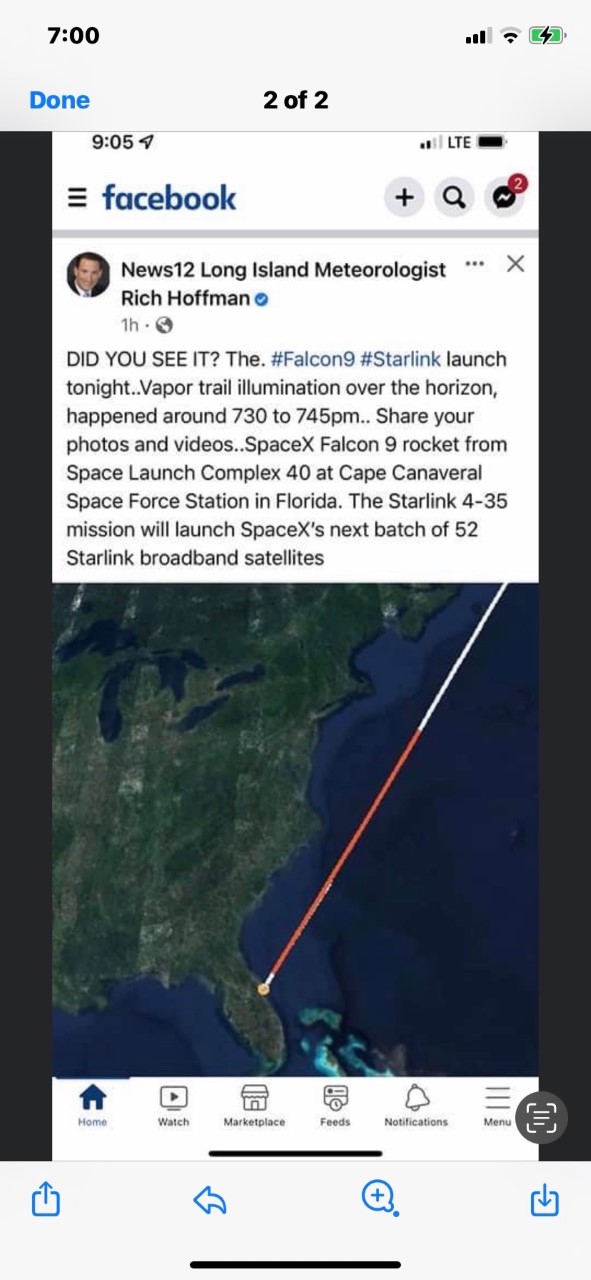
Homework tonight: Matter HW#1 for Tuesday.
Make ups for the first Measurement Celebration available by period 1 on Tuesday until Friday 300 PM. If you need a make up, get going. You’ll get your celebrations back Tuesday, then study up and hurry up!