What did we do today? What’s coming up?
Friday
Bubble Gum Lab, or finding the percent composition by mass of sugar in bubble gum. You get a piece of gum, and you mass it on the scale. Then you chew the heck out of it (and absorb all that tasty sugar. Every 25 chews you will stop and put it on the scale. The mass of the gum diminishes with chewing, because in your mouth the sugar dissolves into your saliva (water) and you eat it.
After a while the gum stops changing mass because you have absorbed it all. Usually at this point you spit it out and grab another piece.
Since you measure a lot, you can fill in the formula to calculate the percent composition by mass of sugar in your gum, this way:
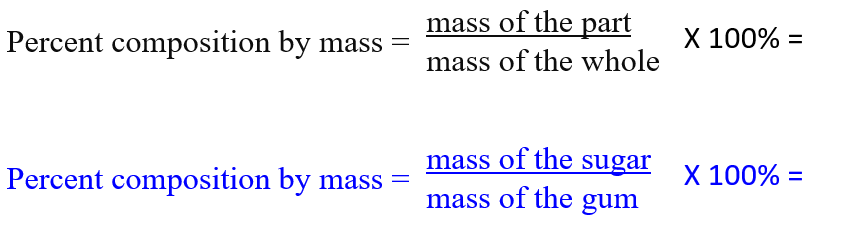
Thursday
Percent comp by mass. All about the proportions; the mass of the part divided by the mass of the whole (times 100%). Formula on the back of the reference table. Simple, just do the molar mass on the left side of your page, then do the percent comp by mass on right.
For example, for water, the molar mass is at left, then the percent comp by mass for H and O are at right. All water is 11% hydrogen BY MASS, and is 89% oxygen BY MASS.
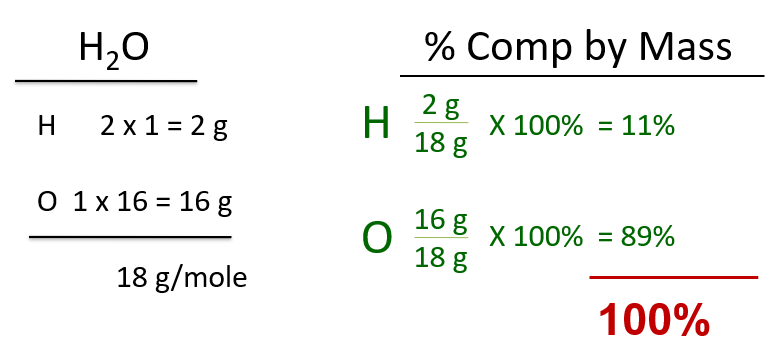
Wednesday Nov 2
Mole Lab: We measured and measured, and then we calculated and calculated. Grams to moles, moles to atoms, or moles to liters. Or atoms to moles, then moles to grams, etc. Molar Mass and more molar mass. Molar mass of elements, and molar mass of compounds. Pentahydrate! (oh my!) Great lab, and we ate Oreos. Nails of iron and nails of aluminum, foil and water, and math until we felt a bit beaten down, then, rising, like a Phoenix, wow.
And, importantly, and understated here, luckily no one was eaten by the Mole Shark. Whew!
Tuesday Wed Nov 1
Mole Islands (welcome to the Club). We hopefully see now the central place of moles, when looking at particle number, molar mass, and volume of gases (at STP). This island map, and it’s “tolls” remind you to always find your place on the map, and figure out your destination island, and use the tolls to guide you through the math.
Monday Halloween Oct 31
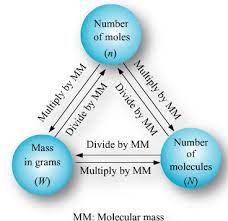
We are making real progress with the idea of MOLAR MASS (the mass of a mole of an element or a compound). Molar mass is AKA gram formula mass (molar mass of an ionic compound), or gram molecular mass (molar mass of a molecular compound).
We’ve seen that grams can be converted to moles, and back again. We’ve also seen that moles can be converted into atoms (or molecules, or F.U.’s!) and back. I hinted today that moles can also be converted to liters of a gas at STP (what’s STP? Look at the title to table A). Only gasses for volume.
Furthermore, I told you that Tuesday you would learn why there are so MANY SHARKS in the classroom artwork. Tomorrow you join the club too.
Friday Oct 28
Ms. Marrin took her turn teaching today, she started the topic of Moles.
A mole is a word that means a number of things. A dozen is 12, a pair means 2, and a mole means 6.02 x 1023 things. That’s a huge number but atoms and molecules are crazy small, you need that many to appreciate them.
We’ll see Monday that a mole is other things too, like a mass, or even a volume. It’s pretty neat.
Thursday Oct 27
Today was review of Naming Compounds X100! We did the “master set” of compounds, truly tricky ones, to make your mind work hard. We also played the naming compounds game, you and a partner. You were the “teacher” or the “student”. Both of you learned. Monday is our celebration of knowledge. It includes making a “T” chart, to determine what compounds are possible and which are not, using the selected oxidation numbers. I will mail you a page with 2 practice problems to try, then check. Click here to see the 2 questions and answers.
Tuesday and Wednesday Oct 25 and 26
Two solid days of review, we did the Names to Formulas practice slides, and the opposite, the Formulas to Names slides as well.
You should be reading your BASICS, drilling this down.
Celebration is Monday, Oct 31 (Halloween)!
Monday Oct 24
Today we covered molecular compound formulas and names. We can only use nonmetals bonding to other nonmetals in molecular compounds. We use the prefix naming method, and use CO, CO2, and H2O as “models” of the naming rules.
Here is a copy of a student conclusion to the electrons lab. It’s a model of what you could be doing, should be doing, etc.
In this lab we used refractive lenses to see the spectra lines of a few gases. . We recorded these spectra lines on paper, and use these colors to identify two “unknown” gases.
Mr. Arbuiso added some flammable alcohol to 6 dishes filled with different salts. He burned the alcohol on them, and we recorded the flame’s spectra colors. Using these spectra colors we identified the salts in 3 different aqueous solutions by burning them in a flame test.
Spectra is produced when electrons go from their excited state back to the ground state. Spectra are unique to each kind of atom and compound, because every atom and compound take different amounts of energy to excite, they emit this exact unique amount to return to the ground state.
Doctors use spectra to see if anything harmful or unusual is inside someone’s body. Astronomers can use refractive lenses to see a distant planet’s spectra, and compare it to already known spectra to see what is on that planet.
It’s had to use flame tests to accurately determine all of the salt solution spectra, because the flames cannot be “broken down” with a refractive lens, because the fire jumps around too much. The spectra lines are actually there, but they move too randomly to track with our eyes.
Some commercial applications of spectra used in the real world are neon signs, and fireworks.
I love chem, the end.
Friday Oct 21
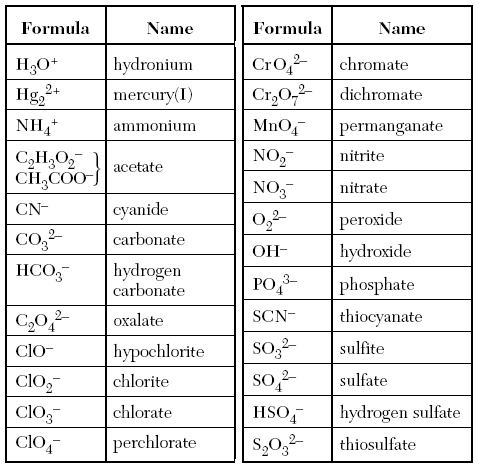
Today is the day we meet Table E (for excellent!). Polyatomic ions are small groups of atoms that cling together and act as ions. There might be 2 or even up to 9 atoms, plus a few extra – or fewer electrons, that bond in the same way that other ions bond, but as a group.
Cations still go first, and anions still go second. The polyatomic ions are in a table, no need to memorize them, but you must familiarize yourselves with them, so that you can recognize them enough to find them on table E.
My favorites include hydroxide (sounds like Hydrox cookies), and hypochlorite (sounds scary but it’s not, unless you are germs!).
We won’t ever have to change their names either, so put your finger into the right box, you’re golden!
Do Naming HW #4 over the weekend please – NOT #3, we’ll get to that next week. Get that Electrons Lab in ASAP, and the Review Lab is overdue now.
Thursday Oct 20
Well, we made it today into the transitional metals! Simple naming of ionic compounds (monoatomic ones) include groups 1 and 2, plus Aluminum on the metal team, and on the nonmetal team we have N, O, F, P, S, Cl, As, Se, Br, and I.
Naming rules are easy enough: say the name of the metal atom first, then say the name of the nonmetal but change it to sound like the -ide name of sodium chloride.
Today we opened up to groups 3-12 (the transitional metals) plus the rest of the metals “under the staircase.”
These metals follow the same exact naming system, except if they can make more than one kind of cation.
Metals lose electrons to become cations. Simple ones lose exactly enough to become isoelectric to noble gases. Some of the transitional metals follow more complicated rules that we don’t have to understand, we just have to deal with. In reality, the electrons are in orbitals which include some hidden from us “sub orbitals”, and the electrons can rearrange themselves into very comfortable and stable arrangements that don’t follow the simple isoelectric rule.
No biggie, just know this: if there are more than one positive number in the box on the Periodic Table, those are the possible cations this atom can form into. Titanium can be either Ti+2 ,or Ti+3, or Ti+4.
These titanium atoms can lose either 2, or 3, or even 4 electrons, depending upon the chemical circumstances. The left over electrons get stable in their hidden sub orbitals and even though they are not isoelectric to noble gases, they work fine.
We name compounds based upon these ion charges.
When Ti+2 cation bonds with O-2, the compound forms is TiO in a 1:1 ratio.
Titanium (II) oxide. The roman numeral matches the Ti+2 ion.
When the Ti+3 cation bonds with O-2, the compound forms is Ti2O3 in a 2:3 ratio.
Titanium (III) oxide. The roman numeral matches the Ti+3 ion.
When Ti+4 cation bonds with O-2, the compound forms is TiO2 in a 1:2 ratio.
Titanium (IV) oxide. The roman numeral matches the Ti+4 ion.
This last one is tricky, if you “criss cross” the ion charges to get 2:4, John Dalton reminds you to make it a simple whole number ratio. So, 2:4 becomes 1:2. Still, since the ion for titanium is +4, the roman numeral name sticks as roman numeral (IV).
![]()
You will only need roman numerals 1 to 7, that’s the most electrons any metal atom might “lose”.
Some transitional metals, like zinc or silver, only have one possible cation state, so NO ROMAN NUMERALS are needed for these transitional metals.
Finally, the roman numeral MATCHES the ion charge, not the formula, not anything else. If there are two ion charges, they may be roman numeral (I) and (II), or as with niobium they are (II) and (V). Not necessarily the first and second, the ION CHARGES matter here.
Wednesday Oct 19
Today was a big day, we learned how to name and how to write formulas for many, many simple ionic compounds. Using 12 metals and 10 nonmetals, we figured out 120 different “mono-atomic ionic compounds”.
Each of the metals in group 1 and group 2 (and aluminum) for cations, positive ions.
Group 1 metals all lose one electron and form only +1 cations.
Group 2 metals all have 2 valence electrons, which they lose when they form into +2 cations.
Aluminum has 3 valence electrons which they “lose” to become only +3 cations.
Metals are said to lose their valence electrons when they become cations. No electrons are “lost” , rather they are transferred to the nonmetals which “gain” them when they form into anions.
Group 17 halogens all have 7 valence electrons, they only gain 1 electron, all forming -1 anions.
Group 16 nonmetals have 6 valence electrons, these must gain 2 electrons to form -2 anions.
Group 15 nonmetals have 5 valence electrons, these gain 3 electrons and form -3 anions.
The cations and anions combine with a perfect transfer of electrons. Each cation must lose exactly the right amount of electrons, and each anion must only gain the exact amount of electrons as necessary.
A neutral ionic compound forms, with a balanced, neutral charge.
Examples include sodium chloride, strontium fluoride, aluminum oxide, potassium sulfide, beryllium nitride, and an odd one, rubidium selenide. Their formulas are: NaCl, SrF2, Al2O3, K2S, Be3N2, Rb2Se.
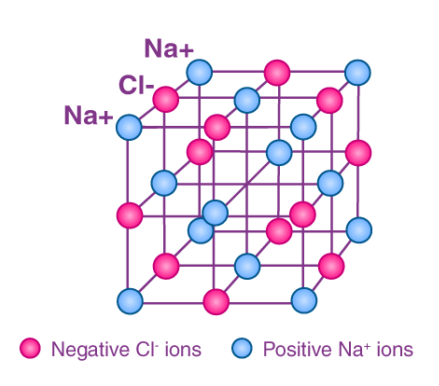
A single F.U. (!) or formula unit of sodium chloride would be a single blue Na+1 cation and a single red Cl-1 anion. A formula unit is the theoretical minimum of an ionic compound you could have, a single unit of the formula. You can never get just “a NaCl” unit, ionic compounds exist as repeating ions in 3D, millions of ions in each direction. But in your mind, you could have just “one” unit.
Tuesday Oct 18
Today we learned what ions are. All atoms are neutral because the number of positively charged protons is always equal to the negatively charged electrons. But in order to bond together, a metal atom will lose an electron (or 2 or 3) and become a positively charged ION. Positive ions are always metals, and get charges like +1, or +2 or +3.
The opposite happens to nonmetals, they are “happy” neutral atoms, but in order to bond to metals, they too must become ions. Nonmetals gain the electrons that are “lost” by the metals. Electrons are never really lost, they just get transferred from metals to nonmetals.
A positive ion is called a CATION. A negative ion is called an ANION.
Metals only lose electrons to become cations (+), while nonmetals only gain electrons to become anions (–)
The bond between a positive cation and negative anion is mad strong. It’s the strongest bond in chemistry, like love.
The positive cations and negative anions must balance to neutral in any compound they form. We have to keep track of the positive ions and negative ions.
Sodium starts as an atom with a 2-8-1 electron configuration. It changes by losing one electron to 2-8. It loses one electron only, and you will know this: a metal must lose only enough electrons to become ISOELECTRIC to a noble gas (group 18). It will end up with the same electron configuration as a noble gas. It will become ISOELECTRIC to the noble gas, but it does not become a gas or a noble gas. It’s just the same as before, but now it’s a charged ion.
Chlorine starts out as an atom with a 2-8-7 electron configuration. It changes by gaining one electron to 2-8-8. It gains one electron only, and you will know this too: a nonmetal must gain only enough electrons to become ISOELECTIC to a noble gas (group 18). It will end up with the same electron configuration as a noble gas. It will become ISOELECTRIC to the noble gas, but it does not become a gas or a noble gas. It’s just the same as before, but now it’s a charged ion.
Metals are positively charged, nonmetals are negatively charged, and then they combine to form into a NEUTRALLY BONDED IONIC COMPOUND. Ionic compounds form from metal cations and nonmetal anions. They make ionic bonds and end up neutral compounds.
Na+1 and Cl-1 form into NaCl in a 1:1 ratio. one +1 ion per one –1 ion sums to neutral. That is why table salt is in a 1:1 ratio, one Na+1 plus one Cl-1 ion makes the NaCl ionic compound.
Oh! And the Boys’ Soccer team won the big STAC game last night!

Monday Oct 17
Today is our Celebration Day, and it happens to be National Brownie Day, which I am celebrating with you, ala Duncan Hines.

The next topic is Naming Compounds, which we start on Tuesday. It’s a few rules, and a lot of drilling to get it organized in your brains. Fun, but details everywhere.
I am going to start calling your parents tonight. Get your work handed in, especially the graded lab reports. Don’t wait, act now!