The point of this lab is to use the concept of percent composition by mass of water in a hydrated ionic compound to discover which compound is our unknown in lab.
You will measure our any amount between 2.50 and 5.00 grams, into an evaporating dish. After 20 minutes of heating, and then five more minutes of cooling, the dish can be put on the scale for the first time.
Reheat it for 4 minutes, then cool it again to get the mass again.
Final Mass is the lowest mass that you get (which should be the second mass)
While it’s heating and water is evaporating away, do the math.
For each of the 7 possible listed hydrated compounds you are to calculate the molar mass and then the percent composition by mass of each part. Do the water as a “unit”, but each other atom is separate from the others. Your work should look like this…
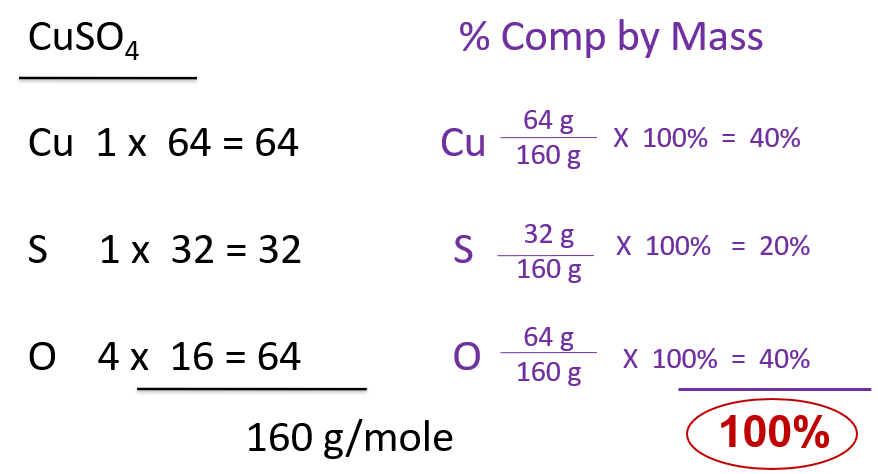
Molar mass on the left hand side of the page, percent comp by mass on the right.
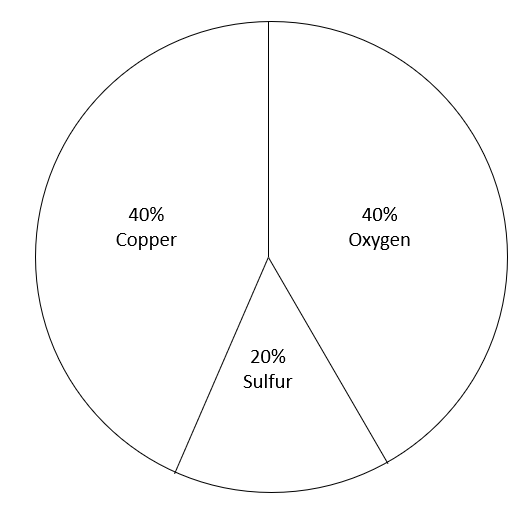
What does that mean? For this compound, CuSO4 (not hydrated) it means this….
At the end of heating and weighing in lab, you should be able to calculate your measured percent comp by mass of water in your lab compound. Use the percent comp by mass formula (back page of reference tables).
The mass of the missing water divided by the total grams you started with, times 100%.
Compare this measured percent comp by mass to the seven values that you calculated in the questions. Your lab percent comp by mass should closely match just one in the seven.
READ the whole lab report, there are a few oddities in there, but they are EASILY EXPLAINED. If you don’t get it, ask. Don’t ever just give up or say ??? That means you want to lose points for not trying.