A funny pick sent to me by Evan F. that is only funny if you are smart.
A funny pick sent to me by Evan F. that is only funny if you are smart.
The best jokes are like that, you can laugh at them, and laugh at those who don’t get it too. (haha)
I am going to keep posting to this post, to make a list of reminders for you, to keep in mind for the regents exam. Nothing in any order, just what comes to me when I am doing my laps.
- Radioactive strontium is bad stuff. It can form from the fission reactions in nuclear reactors. It has a half life of 29 years, give or take and that’s too long to be safe. Why is this stuff so bad? Well, a quick look at table J (for Janet) has Sr higher than Ca. Where is the most calcium in your body? In your bones. What if Sr-90, the bad stuff were to get inside of you, where might it go? It would replace the calcium in your bones, and get stuck in them, and start releasing beta particles into your bone marrow, likely leading to leukemia. (ouch).
- The inner transitional metals are all in group 3. Only group 3. And they fit into period 6 and period 7.
- Vapor pressure is the extra pressure in a closed system that is caused by the evaporation of a liquid. Every liquid has different “strength” intermolecular attractions (electron dispersion, dipole, or even hydrogen bonding), so each will evaporate at its own rate. Hotter makes liquids evaporate faster. A low vapor pressure is due to strong intermolecular attraction. High vapor pressure means the liquid evaporates easily, because it does not have strong intermolecular attractions.
- Isotopes are atoms that are chemically identical, but have a different mass which gives a different atomic mass. They can be naturally occurring, or formed during scientific nuclear reactions.
- Isomers are most often organic compounds that are like LEGOS. You can put them together in one way, with certain functional groups, and properties, or you can put them together in a different way, with different functional groups and different properties. To avoid making problems for yourself, in organic chem we mostly use the condensed structure formulas, rather than standard chemical (or molecular formulas).
For instance, if you had 2 carbons, six hydrogens and one oxygen bonded together, as C2H8O, what would you have? It might be CH3-O-CH3, or it might be C2H5OH. (that’s dimethyl ether or ethanol (ethyl alcohol). The chemical formula gives no hints, but the condensed structural formula shows immediately what compound you might have. - Allotropes (the third of 3 funky vocab words) are pure forms of an element, with different physical bonding, and therefore different properties. Examples are CARBON (sheets of graphite, densely bonded diamonds, and Bucky balls which are carbon “spheres”) and OXYGEN (O2 oxygen to breathe and combust, or O3 ozone, to protect us from harmful rays of the Sun).
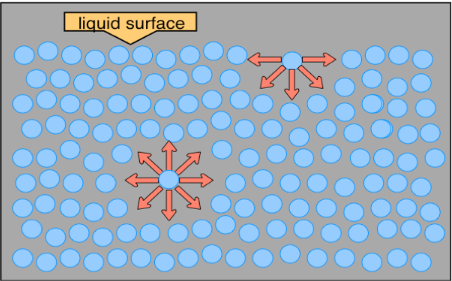
- Hydrogen bonding in water…
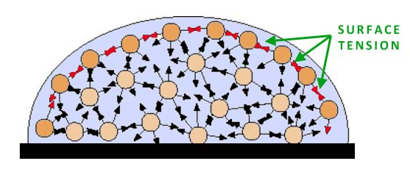
at the surface the water molecules (orange balls here) attract to each other, down and side to side. This creates a “surface”, or edge of water. “INSIDE” the water the hydrogen bonding goes in every direction, there is “no edge”. It’s different at the surface than “inside” the bulk of the water. On the surface bugs (or sulfur) can sit, even though water has a lower density. At the surface it is density vs. density, but the water has this other special “strength” called surface tension. - Here’s another picture to help you “see” this surface tension better.