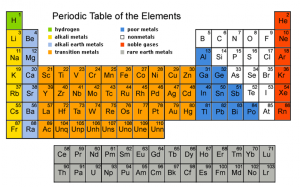
The periodic law, on page 124 in the (awful) textbook, reads:
“When the atoms are arranged in order of increasing atomic number, there is a periodic repetition of their physical and chemical properties (in the groups)”
What that means is this…
The atoms on the periodic table are arranged by order of the number or protons (the atomic number) from 1 to 118. Because the table is in such a funky shape (on purpose) atoms that fall (say) in to group 1, lithium, sodium, potassium, rubidium and cesium, all have similar properties. Their properties “repeated” in group 1.
Atoms in group 17, the halogens, all make negative one anions, all are HONClBrIF twins, all bond similarly, because THEIR properties repeated in group 17.
If the table were just a square or rectangle, their physical and chemical properties would all be exactly the same, but the the table would offer no guidance in what atoms were similar, and which were different.