
Questions have arisen in class about how to convert molar mass values into atomic mass values. This came up with both of the Copper and the Magnesium hydrate labs.
In the copper lab, the question was what is the mass of 5 moles of
CuSO4 5H2O. Then what is the mass of 5 formula units of CuSO4 5H2O
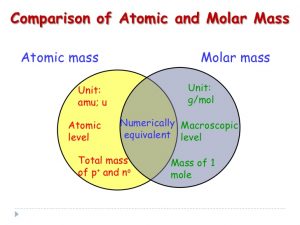
The molar mass calculation should be easy now. The numbers you use for copper, sulfur and oxygen (and for water as H and O) are 64, 32, 16, and 18. Those numbers are the ATOMIC MASSES for each of the atoms. We convert them to “grams per mole” for molar mass.
The math to do this is complicated and not necessary, but here it is… You need a conversion factor, which is: 1.00 gram = 6.02 x 1023 amu
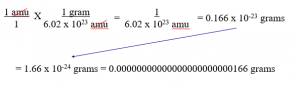 That shows that 1 amu = 1.66 x 10-24 grams
That shows that 1 amu = 1.66 x 10-24 grams
This is a number we try to avoid, for obvious reasons (10-24 of them!)
One mole of CuSO4 5H2O has mass of 250 grams.
How can one mole of this have that mass?
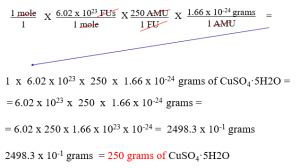
We don’t do the math to go from amu to gram, or the reverse. We just “know” it and that makes our class easier than “real” chemistry you’ll do in college.
If you don’t get this, don’t worry, it’s not part of the course. This is the “proof”.
The photo below is from Natalie in 6th period. A great mole drawing!
